7 Indian Companies Burning Midnight Oil to Develop Covid-19 Vaccines

As of November 24, the global death toll surpassed 14 lakhs amid more than 6 crore cases. Over 4.1crore people have recovered from the disease worldwide.
In India alone, the coronavirus count has surpassed 92 lakhs with a jump of 44,276 cases in the last 24 hours, according to the government data. 489 deaths in the last 24 hours have pushed the total death count to 1,34,743 while 86lakh people in the country have recovered since the beginning of the pandemic. The country has been reporting a steady decline in its daily Coronavirus infections after reaching a peak of about 90,000 cases a day in September. India, which reported its first case on January 30, is the second worst-hit country in the world by the pandemic after the United States.
{this is taken from https://ourworldindata.org/how-to-embed-charts}.
What is a vaccine and understanding its trial stages?
A vaccine is a biological substance type of medicine that stimulates your immune system to produce antibodies to fight with the particular disease but the disease has not come into contact with it before. Vaccines are designed to prevent disease, rather than treat disease once you have caught it.
Phase 1vaccine trials primarily test the vaccine’s safety, determine dosages and identify any potential side effects in a small number of people. Phase 2 trials further explore safety and start to investigate efficacy on larger groups. The final stage, Phase 3 trials, which few vaccines ever make it to, are much larger, involving thousands or tens of thousands of people, to confirm and assess the effectiveness of the vaccine and test whether there are any rare side effects that only show up in large groups.
Why world is racing to discover a vaccine
With more than 6 crore cases and around 14lakh deaths globally, Covid 19 has been described as the first modern pandemic. The vast majority of people are still vulnerable to coronavirus and it is hard to predict how many deaths would ensue if the virus spread continues. The question became even more important this month when the second wave hit European countries and Russia hardly with cases spiking more than ever and countries arere-imposing lockdowns and restrictions.
Countries like India cannot effort the second wave of Covid-19. The unavailability of hospital beds, shortage of medical supplies and staff, together with a sharp contraction in our economy has already hit the country hard.
To counter this, Indian companies are working to get a vaccine out quickly, affordably, and make it available to the entire population.
Status of all vaccines being developed in India
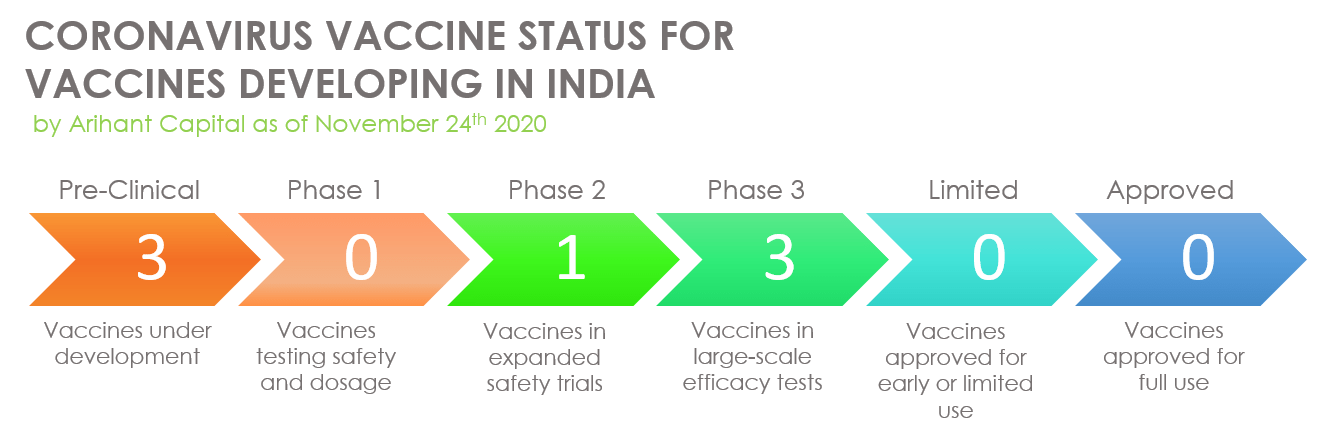
According to the Union Ministry of State, which informed last month, the government has supported more than 30 vaccine candidates for Covid-19 and three of them are in advance phase 2/3 clinical trials now, while four are in the advanced pre-clinical development stage. Below is the list of all the vaccine being tested in India:
COVAXIN
One of the world’s biggest producers of vaccines is now geared up for developing Covid-19 vaccine in collaboration with the Indian Council of Medical Research (ICMR) & the National Institute of Virology (NIV).
COVAXIN by Bharat Biotech International Limited was the first vaccine to received DCGI approval for Phase I & II human clinical trials and the trials begun across India from July 2020. Around 400 people have been selected so far in top hospitals across the country for conducting the trials. After successful completion of Phase 1 and 2 clinical trials of COVAXIN, Bharat Biotech received DCGI approval on Oct 2nd for Phase 3 clinical trials. On 22nd Nov Company said Covaxin will be at least 60% effective based on earlier trial results.
Bharat Biotech already began its Phase-3 clinical trial earlier this month with over 25000+ volunteers across more than 20 cities of India. The company is expected to launch the vaccine in June 2021 with a production capacity of 50 crore doses per annum.
ZYCOV-D
Zydus Cadila, listed Indian bourses, completed its Phase I trials in the month of August, where the vaccine was found to be safe, immunogenic, and well-tolerated in healthy participants. The vaccine was able to elicit a high level of neutralizing antibodies in animal studies.
On the 6th of August, it commenced Phase 2 clinical trials on more than 1000 volunteers, for which the data will be available by the end of November month. The company is planning to conduct Phase-3 clinical trial in the month of December. If the trials of the vaccine remain successful then the company will plan to scale up its vaccine production capacity to 10 crore doses per annum.
COVISHIELD
Pune-based Serum Institute of India (SII)is currently the world’s largest manufacturer of vaccines by volume, with a production capacity of 1.5 billion vaccine doses annually mostly for developing countries. SII is the manufacturing partner of the COVID-19 vaccine named Covishield.
Currently, SII is conducting phase 3 trial with the Indian Council of Medical Research (ICMR) for the Oxford-AstraZeneca vaccine. The firm said it plans to invest $800 million to find, and then produce the COVID-19 vaccine, and has already spent $300 million of that. SII can produce 700-800 million (70-80 crore) vaccine dosages every year once things are streamlined.
The company said India may get COVID-19 vaccine by January 2021 for its emergency use and by April 2021 for general people provided the regulators fast track the process. Chief Executive Poonawalla said 90 percent of Serum Institute’s doses were to be sold to the Indian government at around ₹250 and 10 percent in the private market at a higher price of ~₹500-600. Poonawalla also said the effectiveness of the vaccine is 70 percent with a single dose and a tweak in the dosage helped the effectiveness increase to 90 percent.
SPUTNIK-V
Russia-based Gamaleya Research Institute of Epidemiology and Microbiology has developed Sputnik, are registered vaccine after completion of Phase-1 and Phase-2 trial. Clinical trials for phase-3 of Sputnik V have been announced and have kickstarted in Russia, UAE, India, Venezuela, and Belarus. In September month Indian pharma company Dr. Reddy Laboratories and RDIF agreed to conduct clinical trials of Sputnik V and oversee its distribution in India.
Under the partnership, RDIF shall supply 100 million (or 10 crore) doses of the vaccine to Dr. Reddy’s upon regulatory approval in India. In mid-November, Dr. Reddy received the first batch of doses for conducting Phase 2-3 clinical trial in India. Last week Gamaleya Research claimed that its vaccine is 95 percent effective and will distribute at a cost of around US$20 for its two doses.
ANTISERA
Hyderabad-based Biological E collaborated with ICMR for developing a novel COVID-19 vaccine called Antisera which recently completed its pre-clinical trial and now. Recently, the Drugs Controller General of India has given permission for conducting Phase-1 and Phase-2 human clinical trials which may begin from next month. ICMR said it was developed by injecting inactivated SARS-CoV-2 in horses and can be a potential treatment for COVID-19.
Indian Immunologicals Limited
Indian Immunologicals, a subsidiary of the National Dairy Development Board (NDDB), has inked an agreement with Australia’s Griffith University to develop a vaccine for Covid-19. The vaccine is expected to provide long-lasting protection with a single dose. As per the last update, the company is in a phase of pre-clinical trials and research is still going on.
Mynvax
Bengaluru-based pharmaceutical startup Mynvax is developing a heat-tolerantCovid-19 vaccine that can be stored at 37 degrees Celsius. This development could be a game-changer for India which lacks sufficient cold chain facilities. Also, it uses a very different approach involving a protein-based vaccine. The same has been done in collaboration with the Indian Institute of Science (Bengaluru). As per the latest update, scientists have completed its pre-clinical trials and now in talks with the manufacturer to take formula design and to start a human clinical trial. The startup is expecting to launch a safe and effective vaccine in about the next 18-20 months. For further research, the company has applied for a Rs 15 crore grant from the Biotechnology Industry Research Assistance Council (BIRAC).